- Home
- About Us
- TSPT Academy
- Online Courses
-
Resources
- Newsletter
- Business Minded Sports Physio Podcast
- Day in the Life of a Sports PT
- Residency Corner
-
Special Tests
>
-
Cervical Spine
>
- Alar Ligament Test
- Bakody's Sign
- Cervical Distraction Test
- Cervical Rotation Lateral Flexion Test
- Craniocervical Flexion Test (CCFT)
- Deep Neck Flexor Endurance Test
- Posterior-Anterior Segmental Mobility
- Segmental Mobility
- Sharp-Purser Test
- Spurling's Maneuver
- Transverse Ligament Test
- ULNT - Median
- ULNT - Radial
- ULNT - Ulnar
- Vertebral Artery Test
- Thoracic Spine >
-
Lumbar Spine/Sacroiliac Joint
>
- Active Sit-Up Test
- Alternate Gillet Test
- Crossed Straight Leg Raise Test
- Extensor Endurance Test
- FABER Test
- Fortin's Sign
- Gaenslen Test
- Gillet Test
- Gower's Sign
- Lumbar Quadrant Test
- POSH Test
- Posteroanterior Mobility
- Prone Knee Bend Test
- Prone Instability Test
- Resisted Abduction Test
- Sacral Clearing Test
- Seated Forward Flexion Test
- SIJ Compression/Distraction Test
- Slump Test
- Sphinx Test
- Spine Rotators & Multifidus Test
- Squish Test
- Standing Forward Flexion Test
- Straight Leg Raise Test
- Supine to Long Sit Test
-
Shoulder
>
- Active Compression Test
- Anterior Apprehension
- Biceps Load Test II
- Drop Arm Sign
- External Rotation Lag Sign
- Hawkins-Kennedy Impingement Sign
- Horizontal Adduction Test
- Internal Rotation Lag Sign
- Jobe Test
- Ludington's Test
- Neer Test
- Painful Arc Sign
- Pronated Load Test
- Resisted Supination External Rotation Test
- Speed's Test
- Posterior Apprehension
- Sulcus Sign
- Thoracic Outlet Tests >
- Yergason's Test
- Elbow >
- Wrist/Hand >
- Hip >
- Knee >
- Foot/Ankle >
-
Cervical Spine
>
- I want Financial Freedom
- I want Professional Growth
- I want Clinical Mastery
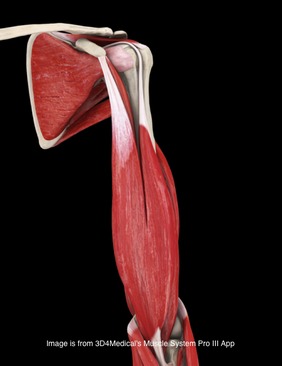
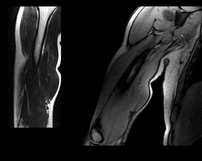 Background Information: Biceps Tendinitis encompasses a spectrum of disorders including primary biceps tendinitis, patients without primary tendinitis, and biceps tendinosis. Primary Biceps Tendinitis (5%) is inflammation of the biceps tendon in the intertubercular groove caused by mechanical stresses and overuse. Some clinicians identify biceps tendinitis simply by individuals who exhibit anterior shoulder pain that is exacerbated by shoulder and elbow flexion (Mitra et al, 2011). Mechanical stresses can include entrapment, instability, and spontaneous rupture of the long head of the biceps tendon. These patients often suffer from secondary impingement because of scapular instability, anterior capsule laxity, or posterior joint capsule tightness. Patients suffering from secondary impingement are often your younger athletic population between 18-35 years old. Primary tendinitis is much less common than those without primary tendonitis (95%). The patients without primary tendonitis have biceps tendon pathology secondary to a rotator cuff injury or labral tear. Interestingly, biceps tendinopathy is rarely found alone. It usually occurs secondary to or a cause of another pathology (Zhang et al, 2011). 85% of patients with long head of the biceps tendon partial tears have associated rotator cuff pathologies (Gazillo 2011). Additionally the umbrella term biceps tendinitis includes biceps tendinosis, which is caused by primary impingement of the shoulder and overuse. This usually involves your older population >35 years of age. Due to their age and primary impingement, these patients are also at risk for rotator cuff pathology. A healthy biceps tendon is "characterized by a hierarchical arrangement of collagen fibers and in tightly packed parallel bundles" (Buck 2009). Tenocytes, which produce collagen molecules, fibroblasts, and stainable ground substance are absent in a normal biceps tendon. The Nho article discusses a nice algorithm of the progression from Early tenosynovitis (inflammation) to early tendinosis (fibrosis) to late stage tendinosis (degeneration). Throughout each stage there is a shift in the cellular and collagen matrix altering how the patient will present to your clinic. (See Reference below) Anatomy: Origin: Apex of coracoid process of scapula (short head); Supraglenoid tubercle of the scapula (long head) Insertion: Tuberosity of the radius and aponeurosis of the biceps brachii (lacertus fibrosis) Action: Flexes the shoulder joint. The short head assists with shoulder adduction. The long head may assist with abduction if the humerus is laterally rotated. With the origin fixed, flexes the elbow joint, moving the forearm toward the humerus and supinates the forearm. With the insertion fixed, the biceps muscles flexes the elbow joint, moving the humerus toward the forearm, as in pull-up or chinning exercises. Innervation: Musculocutaneous nerve (C5-6). *(Kendall et al, 2005) Pathophysiology: Knowing the anatomy of the Biceps Brachii can be useful for understanding the pathologies involved with the muscle. While the long head does attach to the supraglenoid tubercle of the scapula, it also attaches to the superior labrum. This can potentially contribute to labral injuries and explains why some tests like the Biceps Load II are able to assess the labrum. Due to its attachment to the labrum, the long head of the biceps tendon is highly innervated, containing substance P and calcitonin gene-related peptide (Ryu & Pedowitz, 2010). These play a part in vasodilation, plasma extravasation, and pain transmission. While the majority of the long head of the biceps tendon is supplied by the anterior humeral circumflex artery, recent studies have shown that there is relative avascularity in the region of the superior glenoid (Nho 2010). The long head is extrasynovial, but due to it's proximity to the synovium, the inflammatory process that is involved with the rotator cuff can spread to the biceps as well (Pill et al, 2012). Upon visual inspection during rotator cuff surgery, the long head of the biceps tendon is often found to be inflammed. Some researchers proposed the idea that the depth or shape of the intertubercular sulcus would influence biceps tendon pathology, but Abboud et al found no correlation when studying shoulder morphology via MRI. Additionally, the 9 cm-long biceps tendon travels between the lesser and greater tubercle in the intertubercular sulcus. It has been found to be round/cord-like in the sulcus and wide/flat in the intra-articular portion. As the tendon exits the sulcus, it makes a 30-40 degree turn towards the supraglenoid tubercle (Elser et al, 2011). This inflammation can be further explained because the long head of the biceps tendon resides more on the medial wall of the sulcus, creating repetitive stresses with overhead use. The tendon is supported in a tendoligamentous sling including the coracohumeral ligament, superior glenohumeral ligament, subscapularis tendon, and supraspinatus tendon. All contribute to forming a protective covering over the intertubercular sulcus. Additionally, the falciform ligament has been shown to attach to both sides of the bicipital groove, further supporting the tendon (Nho 2010). This increases the stability of the biceps tendon, lowering the chances of subluxation. Although previously misunderstood, it is now known that the transverse humeral ligament does not aid in supporting the tendon within the intertubercular groove (Synder 2012). Due to the long head of the biceps tendon crossing the glenohumeral joint, it has been theorized that the tendon contributes to stability at the shoulder as well. The primary stabilizers of the shoulder, the rotator cuff muscles, have a few regions at which they are unable to protect the shoulder, one of which is the rotator interval. Fortunately, the biceps tendon and coracohumeral ligament reinforce this area. Based on its positioning, the long head of the biceps tendon has been theorized to protect against superior and anterior translations as well (Neumann, 2010). Others have thought that the LHB acts as a humeral head depressor as well (Nho 2010). In studies where the long head of the biceps tendon was cut, superior migration was noted with the humerus relative to the scapula, especially during abduction movements (Elser et al, 2011). Pill et al also found the long head of the biceps to be a more powerful supinator of the forearm compared to the short head, and the short head was found to be a more powerful elbow flexor compared to the long head. According to Goldman, a related pathology is calcific tendinitis of the long head of the biceps. Calcific tendinitis is thought to result from excessive compressive forces from adjacent tissues. It has been found to be most common at the proximal myo-tendinous junction of the long head of the biceps and near the supraglenoid tubercle attachment. While it may present as anterolateral humeral pain, it is likely to be masked by other signs/symptoms due to the frequency with which it appears with other shoulder pathologies, such as subacromial impingement, rotator cuff tears, and more. Clinical Presentation: Patients with Biceps Tendinopathy may present with tenderness upon palpation of the long head of the biceps tendon in the intertubercular sulcus, especially when combined with passive external rotation (Pill et al, 2012). The most common clinical finding is palpatory point tenderness in the bicipital groove with the arm in 10 degrees of internal rotation (Churgay 2009). The pain is a deep, throbbing ache localized to the anterior or anteriomedial shoulder. Due to the location of symptoms, differential diagnosis should include rotator cuff tears, AC Joint dislocation, subacromial bursitis, impingement syndrome, adhesive capsulitis, cervical spine pathologies, and more. Pain may occur at night and spread to the biceps muscle belly and into the radial aspect of the hand (Synder 2012). Being able to palpate for the biceps tendon within the intertubercular groove is necessary for proper diagnosis. In a study written by Gazzillo et al, he reported medical clinicians often do not palpate the intertubercular groove with much accuracy. Many clinicians miss medially by 1.4 +/- .5 cm (Gazzillo 2011).* Patients with tendinopathy may report a clicking, popping, or snapping sound with humeral motion due to biceps tendon instability. Many times if tendon instability is noted, partial or full subscapularis tears are also present (Nho 2010 & Synder 2012). Yerguson's Test, Speed's Test, and O'brien Active Compression Test have all been found clinically useful in diagnosing patients with biceps tendinopathy. Again, these tests are also indicative of other shoulder pathology, so use proper clinical reasoning and cluster your findings. The clinical presentation can be strengthened with imaging. Magnetic Resonance Arthrography has shown to be sensitive and moderately specific in diagnosing biceps pathologies. Arthrography has been useful in determining associated injuries as well. MRI can show changes in tendon diameter and signal abnormalities. The sensitivity of tendon diameter changes is in between 59% and 82% and a specificity between 64% and 88% (Buck 2009). Buck et al states that tendon pathology on MRI should be evaluated by 1) diameter changes 2) signal changes 3) equivocal findings on different MRI sequences. To date, the role of ultrasound in diagnosis has not been established; however, due to its ability to visualize with movement, it may prove to be a useful component in the future (Mitra et al, 2011).  Treatment: The traditional approach for treating biceps tendinopathy begins with a conservative method. This may include: NSAIDs, ice, rest, lifestyle changes, steroid injections, stretching program (especially the posterior capsule), and progressive strengthening program of the scapular stabilizers and rotator cuff (Mitra et al, 2011). Once the acute phase has been managed, ROM becomes a focus of therapy with exercises like wall walking, towel-aided stretches, and pulleys. It is important to avoid abduction and overhead activities initially as these stress the tendon too much (Ryu & Pedowitz, 2010). Next, progressive strengthening and stretching exercises are added, especially the posterior capsule. The hamstrings and low back should also be assessed, as subtle losses in range of motion distally can affect mechanics at the shoulder joint (Churgay 2009). For biceps tendinopathy with impingement, the approach is generally the same. Treatment techniques for subacromial impingement are also included, such as manual therapy, decreasing usage of the deltoid (this encourages superior humeral translation), and a focus on the inferior rotator cuff. Treatment will be largely based off clinical and imaging findings. Many individuals believe the ultimate necessary fix for biceps tendinopathy involves a tenodesis or tenotomy. Tenotomy is the procedure of choice in patients over 60 y.o. with a ruptured biceps tendon (Churgay 2009). Tenodesis is more appropriate for patients under 60 y.o. and still very active. The goal with tenodesis is to maintain the length-tension relationship of the biceps muscle. There have been reports of superior humeral migration following these procedures, due to the loss of the depressing effects the long head of the biceps tendon provides. Senturk et al found no clinical, functional, isokinetic or cosmetic difference between the results for patients who received a tenodesis or tenotomy following chronic biceps tenosynovitis. Some practitioners recommend transverse humeral ligament decompression along with synovectomy (Elser et al, 2011). A more conservative treatment may include a steroid injection. The injection should occur in the tendon sheath, not the intratendinous junction (Zhang et al, 2011). To improve accuracy, the injection can be guided via ultrasound or flouroscopy (Mitra et al, 2011). Steroid injections have been shown to decrease pain, and the accuracy can be improved upon via ultrasound guidance. Due to the lack of consistency in the pathological process being in a truly inflammed state, perhaps this diagnosis should not be labeled as an "-itis." The tendon is also found to be in a degenerative state, which is frequently known as an "-osis." It may be more appropriate to label these patients as having tendinopathy (Reinking, 2012). With the success seen with eccentric exercises and other tendinopathies (achilles, lateral epicondyle, patellar), it would be interesting to see the effect eccentric training could have on biceps tendinopathy. Perhaps this is something we will see in the future as research on eccentrics progresses. * Palpating the Lesser Tubercle, Intertubercular Sulcus, & Greater Tubercle: -Have the subjects arm against their body with the elbow bent to 90 degrees. -Palpate with broad fingers over the pectoralis major and clavicular portion of the deltoid. -With your second hand, bring the patient's arm into shoulder external rotation. -In this position, feel the coracoid process beneath your fingers (just outside it will be the lesser tubercle). -Next bring the arm into internal rotation, your fingers will dip into the intertubercular sulcus and then round up onto the greater tubercle. (Tixa 2008) References:
Abboud JA, Bartolozzi AR, Widmer BJ, DeMola PM. (2010). Bicipital groove morphology on MRI has no correlation to intra-articular biceps tendon pathology. J Shoulder Elbow Surg., 19(6):790-4. Web. 10 Dec 2012. Buck F, et al. (2009). Degeneration of the Long Biceps Tendon: Comparison of MRI With Gross Anatomy and Histology. AJR, 193: 1367-75. Web. 10 Dec 2012. Churgay C. (2009). Diagnosis and Treatment of Biceps Tendinitis and Tendinosis. American Family Physician, 80.5, 470-476. Web 6 Dec 2012. Elser F, Braun S, Dewing CB, Giphart JE, Millett PJ. (2011). Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy, 27(4):581-92. Web. 10 Dec 2012. Gazzillo G, et al (2011). Accuracy of Palpating the Long Head of the Biceps Tendon: An Ultrasonographic Study. Physical Rehabilitation and Medicine, 3.11. 1035-1040. Web 7 Dec 2012. Goldman AB. (1989). Calcific tendinitis of the long head of the biceps brachii distal to the glenohumeral joint: plain film radiographic findings. AJR Am J Roentgenol., 153(5):1011-6. Web. 12 Dec 2012. Kendall FP, McCreary EK, Provance PG, Rodgers MM, & Romani WA. (2005). Muscles: Testing and Function with Posture and Pain. (5th ed., p. 290). Baltimore: Lippincott Williams & Wilkins. Mitra R, Nguyen A, Stevens KJ. (2011). Fluoroscopically guided supraglenoid tubercle steroid injections for the management of biceps tendonitis. Pain Pract., 11(4):392-6. Web. 10 Dec 2012. Neumann D. (2010). Kineseology of the Musculoskeletal System: Foundations for Rehabilitation. (2nd ed., pp. 136-140). St. Louis: Mosby Elsevier. Nho S, et al. (2010). Long Head of the Biceps Tendinopathy: Diagnosis and Management. Journal of the American Academy of Orthopaedic Surgeons, 18.11, 645-656. Web. 7 Dec 2012. Pill SG, Walch G, Hawkins RJ, Kissenberth MJ. (2012). The Role of the Biceps Tendon in Massive Rotator Cuff Tears. Instr Course Lect., 61, 113-120. Web. 7 Dec 2012. Reinking M. (2012). Tendinopathy in athletes. Phys Ther Sport., 13(1):3-10. Web. 12 Dec 2012. Sentürk I, Ozalay M, Akpınar S, Leblebici B, Cınar BM, Tuncay C. (2011). Clinical and isokinetic comparison between tenotomy and tenodesis in biceps pathologies. Acta Orthop Traumatol Turc., 45(1):41-6. Web. 10 Dec 2012. Synder G, Mair S, & Lattermann C. (2012). Tendinopathy of the Long Head of the Biceps. Medicine and Sports Science, 57. 76-89. Web 9 Dec 2012. Tixa S. (2008). Atlas of Surface Palpation. (2nd ed., pp. 104). Paris: Elsevier. Zhang J, Ebraheim N, & Lause G. (2011). Ultrasound-Guided Injection for the Biceps Brachii Tendinitis: Results and Experience. Ultrasound Med Biol., 37(5):729-33. Web. 9 Dec 2012.
4 Comments
 Introduction: Low Back Pain is one of the most common musculoskeletal conditions with a lifetime prevalence between 70-85%. Ninety percent of the population will improve, but the other 10% will go on to develop chronic low back pain (cLBP). Known as a "Western Epidemic," it is estimated that the total costs of LBP each year amount to 100 billion dollars in the United States alone (Tsoa 2008). Chronic pain is defined as pain lasting longer than 6 months that is not related to a malignancy. Our current belief of pain roots from the traditional medical model that pain is a "physical response to an organic dysfunction (Wolff 1991)." Throughout the medical community there has been a push to change that traditional thinking pattern. New pain models are being produced with reliable data to support their findings. New Pain Models: One such model is the kinesiopathologic model of pain. This model focuses on altered neural control and cortical feedback as the source of a patient's symptoms. It is no longer simply mechanical tissues that are involved. Since cortical regions are partially responsible for postural control, altered cortical activity could therefore be responsible for altered postural stability. Coincidentally, patients with cLBP often demonstrate postural instability and altered muscle timing. Researchers have speculated that patients with cLBP require a more direct influence of their executive motor systems to perform postural coordination tasks. In healthy subjects, these tasks are automatically controlled by sub-motor systems. Therefore, pain alters how the brain processes postural coordination. Several studies have shown decreased deep abdominal and back muscle activation along with increased superficial muscle activation in cLBP patients (Tsoa 2008). Repetitive posturing and abnormal movement patterns expose the lumbar spine to low load long duration forces, creating abnormal movement. Therefore, rehabilitation of cLBP should include retraining of postural coordination (Jacobs 2010). Delving deeper in the altered muscle timing, Tsoa et al found decreased transversus abdominus (TrA) firing in patients with recurrent low back pain. The study demonstrated how cLBP patient's had poor anticipatory postural adjustments when performing rapid voluntary limb movements. Since anticipatory postural adjustments occur before movement, they must be controlled by the central nervous system. In healthy subjects, there was an appropriate feedforward mechanism, and subjects would involuntarily contract their TrA prior to arm movement. The same was not true for recurrent LBP patients. They demonstrated decreased TrA timing when asked to perform an arm movement.  Imaging of the brain in patients with cLBP has shown plastic changes that have occurred in the gray matter of the thalamus and prefrontal cortex. When confronted with a painful stimuli, these same individuals had increased cortical activation and decreased blood flow to the contralateral thalamus (Kobayashi 2009). Kobayashi found a disproportional exaggeration to painful stimuli, indicating there may be a behavioral characteristic to patients with cLBP. The authors outline a "pain matrix" that exists in patients with cLBP. The matrix includes the prefrontal cortex, insular cortex, posterior cingulate gyrus, supplementary motor area (SMA), and premotor areas (PMA) of the brain. The insular cortex and prefrontal cortex are both important in the affective and cognitive components of pain. Additionally, the SMA and PMA are involved in motor planning. It can be assumed that if these portions of the brain are altered in cLBP, their functions will be altered as well. Instead of promoting positive behaviors, cognition and motor planning focus on protective behavioral responses, trying to avoid pain. Additionally, if we view pain from the neuromatrix approach, it must be understood as a multiple system output. As stated above, it is thought that pain reduces the cortical output capacity, which decreases cognitive functioning and increases error rate. As clinicians this is evident in our chronic pain patients, who are often easily distracted and forgetful. Moseley states that pain decreases the immune response, alters sympathetic nervous system activity, and decreased reproductive system activity. This same article found increased pain activation in the anterior cingulate cortex (ACC) (Moseley 2003). This region is responsible for emotional stability and strategizing motor planning. Consistently, patients had an exaggerated hypersensitivity to stimuli. Small inputs were enough to stimulate the brain and incur pain. With a heightened response and altered emotional control, we can see why these patients are timid to perform their typical ADLs.  How can we treat chronic pain? In order to be successful when treating patients with chronic pain, you must understand their suffering as greater than simply a mechanical issue. You must search for the root of the patient's pain. The mechanism based classification of pain is a system that divides pain into categories based off the tissue/ structures that are involved. This classification divides pain into 5 categories: central sensitization, peripheral sensitization, peripheral nociceptive mechanism, sympathetically maintained, and cognitive-affective mechanism. Based off the particular mechanism that is being considered, the patient will present with specific symptoms. For central sensitization, the somatosensory system is at fault. Neurons and nociceptive pathways are receiving altered information. As clinicians, patients who suffer from this type of pain often report hypersensitivities to light, noise, mechanical pressure, and temperatures. Education, pain relieving modalities, and relaxation are KEY to a successful treatment. The peripheral mechanism is defined as pain "rooting from the peripheral nervous system" (Kumar 2011). These patients will often have positive neurodynamic tests. Treatment includes neural mobilization and nerve massage. The peripheral nociceptive mechanism is the traditional belief of pain that symptoms root from either a mechanical or visceral structure (anything but the peripheral nerves). Pain travels through small diameter afferents via the Gate Control Theory. Clinical presentation includes swelling, redness, pain with movement, and pain with palpation. Treatment includes exercise, manual therapy, stretching, and modalities. Sympathetically maintained pain is often defined as chronic pain that is "out of proportion to the injury." Clinical representation includes autonomic dysfunction (vasomotor changes and inflammatory responses) and deep achy pain that is unrelieved by positions. Treatments include Thermal therapy, TENS, and Sympathetic trunk mobilization. Finally, the cognitive affective mechanism includes the psychological component of pain. Graded exposure, relaxation, and distraction are all beneficial in treating this mechanism of pain. When seeing patient with chronic pain, often more than one of these mechanisms will be at play. Being able to differentiate which mechanisms are the root of the patient's symptoms will allow treatment to be most effective. As we saw above, education is extremely important with these patients. But how do we educate? One systematic review we analyzed considered the effects of Neuroscience Education (NE) with your cLBP patients. NE is a cognitive-based education tool focused on decreasing pain and disability by increasing the patient's awareness of the nervous system. Our initial reactions were that patients would not have the capabilities to understand how the nervous system processes, but studies have reported that this method can have "immediate improvements in pain, cognition, and physical performance" (Louw 2011). The NE session could be administered in as little as 30-45 minutes and integrated with other physical interventions. Common resources used include teaching tools, hand-drawn images, pictures, workbooks, and neurophysiology pain questionnaire. Additionally, the Moseley article suggests a 3 part intervention system that addresses identifying the painful stimulus, targeting components of the neuromatrix (while avoiding a flare up), and gradually introducing new stimuli to increase physical function. These concepts are very similar to what Louw identifies in his neuroscience education approach as explained above. Ultimately, respect the patient's suffering, make sure they understand his/her pain, and establish functional baselines. While Neuroscience Education and other pain specific interventions are important, we cannot forget our core exercises when working with cLBP patients. Many of these patients exhibit altered muscle recruitment of the lumbar multifidi, which are responsible for intervertebral motion. Additionally, they demonstrate increased superficial back musculature activation with movement, which arguably increases spinal loading. One study we evaluated assessed 20 patients with unilateral LBP lasting >3 months. Ten of the subjects were placed in a "skilled intervention group" that involved using cognitive attention to activate the lumbar multifidi (LM). The other 10 subjects were put into an "extension exercise group" that focused on activating all paraspinal muscles together non-selectively. The study found that skilled training allowed for greater activation of the lumbar mulitifidi and reduced activity in the superficial paraspinals. This knowledge can potentially decrease abnormal spinal loading and the patient's symptoms. In conclusion, respect your patients' symptoms. Many of these individuals have had pain for a long time. Their perception of pain is altered, which affects their ability to move properly. Repetitive abnormal movement creates more pain and more disability. Find the root of your patients' symptoms and begin to retrain how they think and move! Here is a nice video that explains pain in patient friendly terms: References:
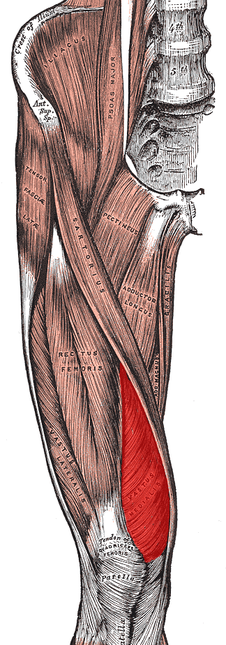
Jacobs J, et al. "Low back pain associates with altered activity of the cerebral cortex prior to arm movements that require postural adjustment." National Institute of Health. 121.3 (2010). Web. 9 Nov. 2012. Kumar S and Saha S. "Mechanism-based classification of pain for physical therapy management in palliative care: a clinical commentary." Indian Journal of Palliative Care. 17.1 (2011). Web. 13 Nov. 2012. Louw, Adriaan, et al. "The Effect of Neuroscience Education on Pain, Disability, Anxiety, and Stress in Chronic Musculoskeletal Pain." Arch Phys Med Rehabili. 92(2011). Web. 11 Nov. 2012. Moseley G. "A pain neuromatrix approach to patients with chronic pain." Manual Therapy. 8.3 (2003). Web. 13 Nov. 2012. Tsoa H, et al. "Reorganization of the motor cortex is associated with postural control deficits in recurrent low back pain." Brain. 131 (2008). Web. 13 Nov. 2012. Tsoa H, et al. "Motor Training of the Lumbar Paraspinal Muscles induces Immediate Changes in Motor Coordination in Patients with Recurrent Low Back Pain." The Journal of Pain. 11.11 (2010). Web. 1 Dec. 2012. Wolff M, et al. "Chronic Pain- Assessment of Orthopedic Physical Therapists' Knowledge and Attitudes." Physical Therapy Journal of the American Physical Therapy Association. 71.3 (1991). Web. 11 Nov. 2012 Yoshitaka Kobayashi, et al. "Augmented Cerebral Activation by Lumbar Mechanical Stimuli in Chronic Low Back Pain Patients." SPINE. 34.22 (2009). Web. 11 Nov. 2012. No matter where you do your rotations or practice physical therapy, you are bound to work with both people who target the VMO with their interventions and people who think it's impossible to do so. Following trauma, knee surgery, or patellofemoral pain syndrome, many practitioners claim selective atrophy and weakness of the VMO relative to the rest of the quadriceps. This becomes a focus of several interventions in the patient's care plan. Due to the controversial state of this case, we thought we would do a review on the isolation of the vastus medialis muscle. Let's start out with reviewing a little anatomy. The quadriceps is made up of four parts: rectus femoris, vastus lateralis, vastus medialis, and vastus intermedius. Rectus Femoris Origin: -Anteroinferior iliac spine (straight head), groove above rim of acetabulum (reflected head) Vastus Lateralis Origin: -Proximal part of intertrochanteric line, anterior and inferior borders of greater trochanter, lateral lip of the gluteal tuberosity, proximal half of lateral lip of linea aspera, and lateral intermuscular septum Vastus Intermedius Origin: -Anterior and lateral surfaces of the proximal 2/3 of the body of the femur, distal half of the linea aspera, and lateral intermuscular septum Vastus Medialis Origin: -Distal half of the intertrochanteric line, medial lip of the linea aspera, proximal part of the medial supracondylar line, tendons of the adductor longus and adductor magnus and medial intermuscular septum Common Insertion: -Proximal border of the patella and through the patellar ligament to the tuberosity of the tibia Common Innervation: -Femoral nerve (L2-4) Common Action: -Extends the knee (rectus femoris also flexes the hip) *(Kendall et al, 2005) One way of analyzing this controversial issue is anatomically. Hubbard et al reviewed the anatomy of cadavers to enhance the interpretation of VMO isolation. The vastus medialis is commonly broken down into two components: vastus medialis longus (VML) and vastus medialis oblique (VMO). It is thought that the VMO is responsible for medial patellar tracking, due to its oblique fiber orientation. In the study, the VMO was unable to be found separated from the VML, meaning the VMO was not found to be a muscle independently. Any attempted separation required destruction of muscle fibers. Upon review of innervation, many studies in the past have come across independent innervation, leading to the hypothesis that the VMO was a separate muscle. However, these nerves have been found to be superficial separations from the femoral nerve leading to distal motor units, sensory contribution from the saphenous nerve, or penetrating innervation for the knee capsule. The authors argue that without isolated innervation, the VMO cannot be activated independently (the patella cannot solely be pulled medially); it is the activity of the femoral nerve the stimulates the entire vastus medialis to contract and extend the knee while pulling the patella medially. Historically, many people were under the impression that the rectus femoris was the prime knee extensor. They thought the vastus medialis was responsible for the final 15 degrees of knee extension due to the inadequacies of the rectus femoris (Lieb & Perry, 1968). Boucher et al looked at the vastus medialis activity in patients with patellofemoral pain syndrome. They found that the vastus medialis and vastus lateralis were not more active in terminal extension. However, they did find that in patients with PFPS, there was a decreased VM:VL ratio compared to the control group. These differences were found to be attributable to a mechanical disadvantage (greater Q-angle); when the Q-angle was decreased, the ratio returned to normal. This same difference in VM and VL ratio was found in another study to be related to mechanical factors as well (Souza & Gross, 2001). Additionally, the authors discovered that isotonic quadriceps contractions elicited larger VM:VL EMG activity compared to isometric contractions. This may influence the treatment plan for patients with pathologies, such as patellofemoral pain syndrome. Ng et al. and Cowan et al. also came across this difference in VM and VL ratio with EMG activity. In fact, the authors were able to normalize the EMG activity with use of therapeutic exercise + biofeedback. Biofeedback has been found to be a useful component in motor retraining, especially regarding earlier activation of the VMO (Cowan et al., 2002). A delay of as little as 5 ms of the VMO relative to the VL can increase the compression forces on the lateral patellafemoral joint (Boling et al, 2006). Powers described a difference between VM and VL activity as well; however, it was attributed to patellar malalignment. Decreased VM activity was not found to be the cause of abnormal patellar tracking or patellar tilt.
As it has probably become obvious, VMO isolation is a controversial issue with many conflicting studies. Thein Brody & Hall do an excellent job summarizing the research, while offering their own opinion. Regarding measuring EMG amplitude, they point out that EMG measures electrical activity, not force production. To truly assess the effects the VMO has on the patella, one must consider fiber orientation, and cross-sectional area. The authors then return back to an anatomical discussion, stating an isolated VMO contraction has never been displayed. This suggests training the VMO independently to improve timing may not be possible. In fact, VMO:VL timing has been shown to improve after training the quadriceps muscle as a whole. So what are your thoughts and what have your experiences been on this issue? Should we be spending so much time trying to isolate the VMO for rehabilitation or focus on a more general method? Let us know! References:
Boling MC, Bolgla LA, Mattacola CG, Uhl TL, Hosey RG. "Outcomes of a weight-bearing rehabilitation program for patients diagnosed with patellofemoral pain syndrome." Arch Phys Med Rehabil. 2006 Nov;87(11):1428-35. Boucher JP, King MA, Lefebvre R, Pépin A. "Quadriceps femoris muscle activity in patellofemoral pain syndrome." Am J Sports Med. 1992 Sep-Oct;20(5):527-32. Web. 17 Nov 2012. Brody LT & Hall CM. (2011). Therapeutic Exercise: Moving Toward Function. (3rd ed., pp. 530-531). Baltimore: Lippincott Williams & Wilkins. Cowan SM, Bennell KL, Hodges PW, Crossley KM, McConnell J. "Delayed onset of electromyographic activity of vastus medialis obliquus relative to vastus lateralis in subjects with patellofemoral pain syndrome." Arch Phys Med Rehabil. 2001 Feb;82(2):183-9. Web. 26 Nov 2012. Cowan SM, Bennell KL, Crossley KM, Hodges PW, McConnell J. "Physical therapy alters recruitment of the vasti in patellofemoral pain syndrome." Med Sci Sports Exerc. 2002 Dec;34(12):1879-85. Web. 26 Nov 2012. Gilleard W, McConnell J, Parsons D. "The effect of patellar taping on the onset of vastus medialis obliquus and vastus lateralis muscle activity in persons with patellofemoral pain." Phys Ther. 1998 Jan;78(1):25-32. Web. 25 Nov 2012. Hubbard JK, Sampson HW, Elledge JR. "Prevalence and morphology of the vastus medialis oblique muscle in human cadavers." Anat Rec. 1997 Sep;249(1):135-42. Web. 25 Nov 2012. Kendall F, McCreary E, Provance P, Rodgers M, & Romani W. (2005). Muscles: Testing and Function with Posture and Pain. (5th ed., p. 420). Baltimore: Lippincott Williams & Wilkins. Lieb FJ, Perry J. "Quadriceps function. An anatomical and mechanical study using amputated limbs." J Bone Joint Surg Am. 1968 Dec;50(8):1535-48. Web. 17 Nov 2012. Ng GY, Zhang AQ, Li CK. "Biofeedback exercise improved the EMG activity ratio of the medial and lateral vasti muscles in subjects with patellofemoral pain syndrome." J Electromyogr Kinesiol. 2008 Feb;18(1):128-33. Web. 17 Nov 2012. Powers CM, Landel R, Perry J. "Timing and intensity of vastus muscle activity during functional activities in subjects with and without patellofemoral pain." Phys Ther. 1996 Sep;76(9):946-55. Web. 25 Nov 2012. Powers CM. "Patellar kinematics, part I: the influence of vastus muscle activity in subjects with and without patellofemoral pain." Phys Ther. 2000 Oct;80(10):956-64. Web. 25 Nov 2012. Smith TO, Bowyer D, Dixon J, Stephenson R, Chester R, Donell ST. "Can vastus medialis oblique be preferentially activated? A systematic review of electromyographic studies." Physiother Theory Pract. 2009 Feb;25(2):69-98. Web. 25 Nov 2012. Souza DR, Gross MT. "Comparison of vastus medialis obliquus: vastus lateralis muscle integrated electromyographic ratios between healthy subjects and patients with patellofemoral pain." Phys Ther. 1991 Apr;71(4):310-6. Web. 25 Nov 2012. Stensdotter AK, Hodges P, Ohberg F, Häger-Ross C. "Quadriceps EMG in open and closed kinetic chain tasks in women with patellofemoral pain." J Mot Behav. 2007 May;39(3):194-202. Web. 26 Nov 2012. |
Copyright © The Student Physical Therapist LLC 2023

 RSS Feed
RSS Feed