- Home
- About Us
- TSPT Academy
- Online Courses
-
Resources
- Newsletter
- Business Minded Sports Physio Podcast
- Day in the Life of a Sports PT
- Residency Corner
-
Special Tests
>
-
Cervical Spine
>
- Alar Ligament Test
- Bakody's Sign
- Cervical Distraction Test
- Cervical Rotation Lateral Flexion Test
- Craniocervical Flexion Test (CCFT)
- Deep Neck Flexor Endurance Test
- Posterior-Anterior Segmental Mobility
- Segmental Mobility
- Sharp-Purser Test
- Spurling's Maneuver
- Transverse Ligament Test
- ULNT - Median
- ULNT - Radial
- ULNT - Ulnar
- Vertebral Artery Test
- Thoracic Spine >
-
Lumbar Spine/Sacroiliac Joint
>
- Active Sit-Up Test
- Alternate Gillet Test
- Crossed Straight Leg Raise Test
- Extensor Endurance Test
- FABER Test
- Fortin's Sign
- Gaenslen Test
- Gillet Test
- Gower's Sign
- Lumbar Quadrant Test
- POSH Test
- Posteroanterior Mobility
- Prone Knee Bend Test
- Prone Instability Test
- Resisted Abduction Test
- Sacral Clearing Test
- Seated Forward Flexion Test
- SIJ Compression/Distraction Test
- Slump Test
- Sphinx Test
- Spine Rotators & Multifidus Test
- Squish Test
- Standing Forward Flexion Test
- Straight Leg Raise Test
- Supine to Long Sit Test
-
Shoulder
>
- Active Compression Test
- Anterior Apprehension
- Biceps Load Test II
- Drop Arm Sign
- External Rotation Lag Sign
- Hawkins-Kennedy Impingement Sign
- Horizontal Adduction Test
- Internal Rotation Lag Sign
- Jobe Test
- Ludington's Test
- Neer Test
- Painful Arc Sign
- Pronated Load Test
- Resisted Supination External Rotation Test
- Speed's Test
- Posterior Apprehension
- Sulcus Sign
- Thoracic Outlet Tests >
- Yergason's Test
- Elbow >
- Wrist/Hand >
- Hip >
- Knee >
- Foot/Ankle >
-
Cervical Spine
>
- I want Financial Freedom
- I want Professional Growth
- I want Clinical Mastery
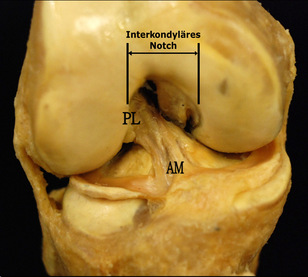
Anatomy The ACL has a medial attachment near the anterior intercondylar area of the tibia and blends with the anterior portion of the lateral meniscus (Markatos et al, 2012). It then ascends in a posterolateral direction to attach to the posteromedial aspect of the lateral femoral condyle. In doing so, the ACL internally rotates as it ascends and is divided into two separate bundles. They are known as the anteromedial and posterolateral bundles. The names are based off tibial insertion sites. With the combination of two bundles and twisting of the fibers, the ACL is able to provide resistance in various angles of knee motion and provides 86% of the stabilization against an anterior drawer force. The anteromedial bundle is taut primarily at 90 degrees of flexion, while the posterolateral bundle increases its tautness as the knee nears full extension. The ACL is able to withstand 1,725 +/- 270 N of force. Since our bodies are impacted by forces greater than this during many athletic activities, the muscles surrounding the knee are able to dampen some of the forces. Based on the orientation of the fibers, the ACL plays a significant role in resisting anterior tibial translation, axial tibial and valgus knee motions. The ACL primarily consists of 90% Type I collagen (10% Type III) and contains many sensory nerve endings, signifying its importance for proprioception and kinesthesia. As the ligament is covered by synovium, it is referred to as extrasynovial, so blood is supplied primarily by branches from the genicular arteries. Mechanism of Injury As parts of the ACL are stressed at each angle of knee motion, the ligament is often at high risk for injury. The majority of ACL injuries are actually non-contact. Three common factors that are associated with ACL rupture include: strong contraction of the quadriceps muscle over a slightly flexed or fully extended knee, a marked valgus collapse of the knee, and excessive internal rotation of the knee (Neumann, 2010). Hyperextension of the knee, while the foot is planted, is also linked to ACL injury. As the ACL limits anterior tibial translation, a strong contraction of the quads will pull the tibia forward and tear the ACL, especially if the hamstring is unable to counteract the force of contraction. Valgus collapse is a phenomenon commonly seen in female athletes. Following landing from a vertical jump or lunge, the femur goes into adduction and internal rotation. The ACL is especially at risk here due to the MCL being lax in a flexed position. Here the ACL plays a larger role in resisting valgus motion. Hyperextension of the knee on a planted foot ruptures the ACL by having the femur slide excessively posteriorly on a fixed tibia. Something to consider is the association between ACL tears and the valgus motion that causes the injury. As a valgus restraint, the MCL plays an important role in protecting the ACL. Often, we see individuals with both ligaments injured. In individuals with sprained MCLs (and thus hypermobile knees), the ACL is placed at increased risk for injury. The MCL is actually one of the prime stabilizers of the knee. Interestingly, the MCL and ACL have different healing positions that contradict one another. If both ligaments are found to be injured, they frequently are repaired during the same procedure. The ideal healing position for the MCL is knee flexion, while the ideal position for the ACL is knee extension. Usually, the ACL's healing is prioritized and the patient has an emphasis placed on gaining extension. This results in the MCL healing into a lax state and leads to hypermobility of the knee, placing the ACL back at greater risk for injury! Perhaps these individuals should undergo one surgical procedure at a time in order to regain a truly stable knee.
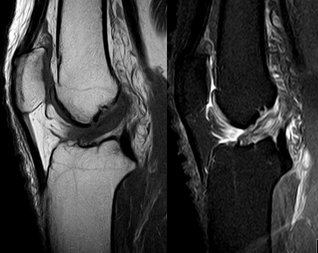
When examining the patient, we will see a loss of flexion and extension, secondary to knee effusion, and quadriceps inhibition. Use of the KT1000 arthrometer has been shown to be accurate in identifying ACL tears with a presence of > 3 mm. difference between sides. Additionally, patients will present with a significant deficit with proprioception due to the ACL's large role in joint positioning. Upon suspicion of ACL rupture, an MRI is often ordered for confirmation. Along with identifying the integrity of the ligament, the MRI can aid in detecting the presence of bone contusions. It is now known that bony contusions following ACL rupture have a strong correlation to the development of knee osteoarthritis. It is for this reason, that we must address the joint effusion quickly. Van Ginckel et al performed a study looking at cartilage deformation following early return to sports. While no difference was found in cartilage thickness at 6 months post injury, the authors did find structural properties of the cartilage that put the tissue at risk for destruction, potentially leading to early development of knee osteoarthritis. It should be noted that females have been found to be at higher risk for ACL injuries compared to males. The proposed intrinsic factors that contribute to this difference include: increased Q-angle, decreased femoral notch size, smaller ACLs, and increased posterior tibial slope (Sutton & Bullock, 2013). The increased Q-angle places a significant valgus force on the knee and ACL, especially when landing from a jump. In fact, the study found that females that had an increase of 8.4 degrees of knee abduction at initial contact were predictive of ACL rupture. Whether or not there are gender differences in femoral notch size is still under discussion, but a frequent component of ACL reconstruction is simultaneous enlargement of the femoral notch. Some research has shown that females have a thinner ACL at midsubstance, but there is also evidence that there is no difference compared to male counter-parts. A posterior tibial slope theoretically places the tibia more anterior to the femur, and upon quadriceps contraction, increases the stress on the graft. There is mixed evidence on this component as well.  Surgical Management: There are almost 200,000 injuries each year involving the ACL, and over half of these require surgical intervention. However, not every individual is appropriate for ACL reconstruction. Those who would benefit from surgery include individuals with repeated episodes of knee buckling, an injury that affected several structures, and the desire to return to a high demand activity that requires knee stability. Factors that may indicate non-surgical management include: little exposure to high demand activities, age > 40 years, severe OA, prolonged coping with ACL deficiency, and those unlikely to comply with post-surgical protocol. The general sequence of surgery is graft harvest/preparation, diagnostic arthroscopy, drill tibia tunnel, drill femoral tunnel, and fixation of graft at each end. The purpose of the diagnostic arthroscopy is to identify any additional injuries in the knee, such as meniscal tears, cartilage damage, etc. Here the surgeon can perform any additional procedures necessary based on the arthroscopy's findings. Next, the physician drills the tibial and femoral tunnels. If using a BPTB graft, the patellar bone is inserted into the tibial tunnel, while the tibial bone is placed in the femoral tunnel (Manske, 2006). For alternate graft types, fixation can be either direct or indirect. Direct fixation includes interference screws, washers, cross-pins, and staples, while indirect fixation devices include suture-posts and polyester tape-titanium buttons. Tunnel enlargement has been found at 5 years after surgery, especially with use of interference screws (Dave et al, 2013). The authors found a biologic component to be involved and offered the opinion that tunnel enlargement could be decreased with accelerated rehab; however, no difference was found in clinical outcomes. Single-Bundle (SB) Technique has long been referred to as the gold standard in ACL reconstruction techniques (Xu et al, 2013). However, as the understanding of the anatomy of the ACL has developed, so has our understanding of surgical reconstruction. The SB has been shown to have good anterior stability but lacking in rotary and frontal plane stability. Since we now know the ACL is important for more than just anterior-posterior stability and with our knowledge of the ACL having an anteromedial and posterolateral bundle, there has been a push to increase utilization of the Double-Bundle (DB) Technique. With the addition of another bundle in the graft, the surgically reconstructed knee can have increased stability in multiple planes as it mimics the dual bundle form of a natural ACL. Xu et al found that DB had greater anterior and rotary stability than SB but no difference in subjective reports of stability. Some of the most common graft selections for ACL reconstruction include: autografts (hamstrings, bone patellar tendon bone (BPTB), quad tendon) and allografts (tib ant, post tib, achilles, BPTB, etc.). The advantage of BPTB include strongest early fixation due to bone healing properties and it doesn't compromise the hamstrings (an important quad force dampener). The disadvantages include direct anterior knee pain, longer early recovery, and length of graft may not match that of tunnels. Some of these disadvantages can be countered by use of a contralateral patellar tendon graft. Ipsilateral grafts have conflicting goals of increasing strength in the quads due to the donor site and increasing ROM in the knee following ACL reconstruction (Manske, 2006). The pros of hamstrings include quicker post-op quad control, easier early rehab, less painful anterior knee pain, and stronger graft. In fact, the hamstring graft can utilize multiple strands, increasing the strength of the graft to greater than all other graft types. Unfortunately, this advantage is somewhat insignificant due to the hamstring grafts having their weakest point at the attachment site. The cons include decreased hamstring activity (hamstrings are important restraint to anterior translation), decreased hamstring strength in deep flexion, graft quality is not as consistent, and the need for suspensory fixation. Allografts are useful because surgery is faster (no need to harvest graft from patient), faster rehab, and more cosmetic. Unfortunately, these grafts are more expensive, place the patient at higher risk for infection, and they don't incorporate as well or fast compared to autografts. Now that we know the theories for selecting each graft type, let's look at some of the recent evidence. The Bone Patellar Tendon Bone (BPTB) graft is commonly referred to as the gold standard in ACL reconstruction due to having 168% strength compared to the ACL and improved bone-to-bone healing properties as compared to ligament-to-bone (Pan et al, 2012). However, BPTB autologous grafts come with the increased chance of donor site morbidity: patellar fracture and anterior knee pain. It was found that artificial ligaments like Ligament Advanced Reinforcement System (LARS) have equal clinical outcomes without donor site morbidity. We may see increased use of artificial grafts in the future. Zelic et al & Abbas et al found there were no significant differences between patellar ligament and hamstring tendon grafts in regards to knee stability except individuals who used a pateller ligament graft had a statistically significant greater chance of developing anterior knee pain at 6 months. However, the anterior knee pain did not affect outcomes. The management of pediatric ACL ruptures is somewhat debatable. The issue of provoking growth deformities by performing transphyseal surgery in reconstruction exists (Frank & Gambacorta, 2013). This led to the approach of delaying surgery until skeletal maturity; however, due to hypermobility without a functioning ACL, the child athletes often experience increased meniscal and cartilage damage (this is also seen in the adult population (Magnussen et al, 2013)). Frank & Gambacorta found improved clinical outcomes with early surgical reconstruction versus delayed for the pediatric population. The potential for growth abnormalities can be decreased by use of soft tissue grafts in transphyseal tunnels. Tissue Healing: Following ACL reconstruction, one of the most important factors that should guide your treatment plan is the tissue healing time frame. As individuals are anxious to return to their prior levels of function, accelerated rehab protocols were developed. Unfortunately, an increase in graft failure rate was noticed and further research was needed to guide the aggressiveness of therapy. It is now known that initially, the graft is actually stronger than a normal ACL and it is the bone attachments in the tunnel that are of concern (Muller et al, 2013). Overly, aggressive activities can loosen these attachments, prolonging inflammatory reactions and leading to graft failure. Controlled motion is encouraged, however, as this can aide in improving some of the biomechanical properties of the graft. After further investigation, we now know that the graft's purpose is merely as a "scaffold" for cell proliferation to occur. There are 3 phases in graft ligamentization: Incorporation, Revascularization, and Graft Healing/Maturation. The time frame is somewhat general, but Incorporation typically occurs in the first 3 weeks. This involves an inflammatory response as the graft degenerates and fibroblasts die. The tissue that remains from this is known as a "scaffold." Revascularization occurs from week 3 to about week 16 as the capillaries invade the graft from the synovium. The host's fibroblasts migrate into the graft tissue. Without this occurring, the graft will fail. At weeks 8-10, the graft is at its weakest point, so it is important to avoid stressing the graft. Graft Healing/Maturation begins after Revascularization. The graft strength drops to as low as 11% of a normal ACL. The graft matures as there is a build-up of collagen content but will not reach normal ACL tensile strength until at least over a year (if at all). According to Rabuck et al, it would appear there may be a role for imaging to be involved with staging of the rehabilitation process in the future. As the ligament undergoes revascularization, MRI can potentially be used to detect the levels of revascularization and thus aid in determining the forces the graft can withstand at the time. CT and radiographs may be useful for detecting widened tunnels at the bony attachments. Rehabilitation: Rehabilitation is often thought of as the time following an ACL reconstruction. With these patients, let us not forget the importance of "prehabilitation"-- the time after injury but before surgery. In fact, there are several reasons surgeons elect to delay ACL reconstruction several weeks following injury. While not all patients receive pre-operative rehab, several studies have demonstrated the importance of a good quadriceps ratio and full knee extension range of motion. Adams et al found that patients with full knee extension ROM, absent or minimal joint effusion, no quadriceps lag with a straight leg raise, and a quadriceps index >90% had better post-surgical outcomes (Adams 2012). The swelling in the knee could impair the healing process following reconstruction and limit ROM. This leads to the next reason for waiting: regaining full ROM. Decreased extension is a significant complication following surgery that may be linked to further knee damage. By regaining the motion prior to surgery, the patient is more likely to return to higher level activity and decrease the chances of further knee injury. The final reason for delaying surgery is to strengthen the quadriceps. As we all know, there is a quadriceps strength deficit following joint effusion and surgery. As quadriceps strength is linked to function, it is desirable to regain as much strength as possible before surgery. Therefore, our goals prior to surgery include decreasing swelling, increasing knee ROM (especially extension) and increasing quadriceps strength. It has been shown that early rehabilitation improves knee function following ACL reconstruction (Zhu et al, 2012). That being said, there are several milestones we should look to reach in rehabbing our patients. Initially, we want to decrease knee pain and swelling, restore knee extension ROM, and improve quadriceps recruitment. These early milestones are similar to our prehabilitation goals. We usually are not the first individuals to jump to using modalities, but evidence shows Russian stim + exercise improves quadriceps strength greater than exercise alone (Kim et al, 2010). Therefore, e-stim may be a useful tool in your rehabilitation program. It's easy to see how important they are for quality outcomes. Once these goals have been achieved, we want to have the patient focus on normalizing their gait. The final items to focus on include restoring knee flexion ROM, proprioception, LE muscle performance, and return to function. These items will be expanded upon later. Following surgical reconstruction, it is essential that the patient achieves full knee extension for maximum functionality. Even a 3-5 degree loss of extension ROM has shown to negatively impact patient subjective and objective reports & an association with decreased quadriceps strength (Adams 2012). Upon failure to achieve full extension, pain with extension overpressure, or an audible clunk with extension, the clinician should consider the presence of a cyclops lesion (Silverwood et al, 2012). It can be confirmed with MRI or arthroscopic visualization. A cyclops lesion is a localized arthrofibrosis in the anterior intercondylar notch and often requires arthroscopic excision. Another reason to achieve full extension, and full ROM for that matter, is to decrease the likelihood of developing OA. Decreased knee ROM following ACL reconstruction has been shown to be associated with the development of knee osteoarthritis (Shelbourne et al, 2012). It is important to remember that "full ROM" refers to normal ROM for the individual. We can use side-to-side comparisons to determine the achievement of full motion.  An important consideration when selecting exercises for your patient is the amount of strain placed on the graft. Escamilla et al found strain on the ACL is greatest at knee flexion angles of 30 degrees and less for both Open Kinetic Chain (OKC) and Closed Kinetic Chain (CKC) exercises, especially at 10-15 degrees of flexion. OKC knee extension at angles < 30 degrees should be avoided until the graft is fully revascularized; however, quadriceps activity at 0 degrees of flexion (full extension) does not overstrain the graft, due to increased stability. This means that straight leg raises (SLR) can be a useful component to strengthening the quadriceps near extension. Be sure your patient does not have any extension lag when performing the exercise, otherwise the integrity of the graft tissue may be put at risk. While the ACL is still strained in CKC, the addition of hip muscle activity and hamstring activity dampen the forces on the graft. These muscles are more active due to the increased need for stability and are more functional than OKC exercises. In higher-level athletes that have returned to pre-injury level, it was found they had greater activation of gluteus maximus and gastrocnemius and decreased activation of the quadriceps (Nyland et al, 2013). As we already know, following ACL rupture the individual experiences decreased knee proprioception. With greater use of the hip and ankle muscles, the patient can increase prioprioception at these joints. Increased sensation and muscle activation at the joints surrounding the knee increases stability in the extremity and puts less stress on the knee (and graft). To further lessen the strain on the ACL during CKC exercises, a forward trunk lean of about 30 degrees can be used. This increases activity of the hamstrings, which we already know counters the ACL strain. When a forward trunk lean is not utilized, we often see anterior knee translation, leading to increased stress on the graft. When the knee travels 8 cm past the toes, the tissue is placed at especially high risk for failure. The review also showed those who used primarily CKC exercises experienced less knee pain during rehabilitation. CPMs are frequently known for their use following total knee arthroplasty, and there has been discussion on their potential for use following ACL reconstruction. Review of the literature has found there is no statistically significant advantage to using CPM following surgery (Lobb et al, 2012). Additionally, there has been further research on the use of bracing. No benefit was found in using bracing following reconstruction, however, it may still play a role for individuals prone to knee stiffness. Bracing can aid in ensuring full knee extension. Interestingly, the review also found no difference between home-based and supervised physical therapy. It's unsure on what level of involvement the therapist should have with home-based PT. Kruse et al also found bracing and CPM to have no benefit at all. You will still frequently see physicians and other health care practitioners prescribe functional braces for athletes following ACL reconstruction. They have been shown to limit only anterior tibia translation only at low loads. The loads in ADLs, cutting, and jumping far exceed that which the brace can protect. While mechanically the brace may have no proven benefit, there may be a role for use if if affects the psychological component of the patient. Kruse et al also found that an accelerated program had no deleterious effects when immediately post-operatively the patients could initiate weight-bearing, knee motion 0-90 degrees of flexion, and CKC exercises. After 3 weeks, eccentric quadriceps and isokinetic hamstring exercises could be included. Eccentric exercises are well known for their utilization with tendinopathy. As quadriceps strength deficits continue to exist for increased lengths of time following ACL reconstruction, therapists are eager to find a method of combating the impairment. While early progressive, high-force eccentric exercises may have been contraindicated in the past, there is evidence that they may be safe to use for these patients and could potentially improve the strength gains of the affected quadriceps muscle (Lepley & Palmieri-Smith, 2012). A potentially useful neurological component to add to the rehabilitation of these patients is cross-exercise. Cross-exercise is training of the same muscle on the uninvolved limb and seeing carryover to the involved limb's muscle. Quadriceps strength in the surgical knee has been shown to be impaired. Due to the previously discussed limitations graft healing places on quadriceps activation, we are unable to focus on the muscle's impairments as much as we would like. In order to rectify the strength deficit, cross-exercise has been shown to improve quadriceps strength and should be considered as an adjunct to your treatment plan (Papandreou et al, 2012). The point in time at which the athlete should return to sport is always a debatable topic. Of use are functional tests (single-leg hop, timed hop test, shuttle run and stair hop test) that compare the involved limb to the uninvolved limb (Baltaci et al, 2012). The role of the psychological component cannot be overstated. Individuals who express fear of re-injury typically do not return to pre-injury level (Feller & Webster, 2013). In fact, using a patient's comfortability with an exercise can be a useful tool for progression. The study showed that individuals who advanced their rehab program based on when they felt comfortable were at no greater risk for re-injury. Check out this previous post by Brian on outcome measures for return to sport! Going back to the issue of gender differences in ACL injuries, an emerging concept to decrease the gap between men and women involves neuromuscular training or movement re-training programs. The theory involves retraining the athletes to avoid vulnerable positions when exercising/competing, especially in one-legged standing, out-of-control baseline landings, and straight leg landings. Strength and power exercises, neuromuscular training, plyometric exercises, and agility exercises should be included. The females are taught to emphasize having their knee over toes when landing from a jump or cutting. Research has shown that these movement training programs can lower the incidence of female ACL rupture to equal that of males (Sutton & Bullock, 2013). To identify individuals who would benefit most from this type of program, a drop off a 31 cm box leading into a full vertical leap can be used. With this test, the examiner can assess knee motions that put the athlete at risk. Another component of ACL injury and re-injury is movement asymmetry. When forced to perform unilateral sport-specific movements, the asymmetries become even more prominent. An important component of injury prevention and rehabilitation should be restoration of movement symmetry (Hewett et al, 2013). Strength symmetry of at least 85% (compared to uninvolved side) is often advised prior to beginning cutting, pivoting, and jumping. This is especially important for quadriceps strength and quadriceps/hamstring ratio. As many of you know, rehabilitation following ACL reconstruction is typically protocol driven. While the protocols differ based on each surgeon, the principles of them are generally the same (see our earlier paragraph on goals). Rehab Webinar's Kevin Wilk has a 6-phase protocol that we have summarized below: -Pre-op: re-establish ROM, control swelling, limit activity, and restore quadriceps control (see our earlier paragraph for increased description) -Immediate Post-op (days 1-7): restore full knee extension and gradually increase flexion (do not overstress!), control swelling, patellar mobilizations, e-stim with exercise, restore quadriceps control and independent ambulation, CKC mini-squats, and ankle pumps -Early Phase (weeks 2-4): focus on hip muscles, HS curls (unless HS graft; then wait 6-8 weeks), cycling, lateral cone high stepping (knees as high as hip), balance beam activity, lateral lunges (starting with 30 degrees of knee flexion and 45 degrees of hip flexion) at various angles), reactive squats, wobble boards, foam, wobble board squats (start as early as 3-4 weeks) progress to one leg with stationary holds and ball toss against rebounder with perturbations Intermediate Phase (weeks 4-10): stabilization muscles from above and below, can progress lateral cones with passing as well and speed, progress lateral lunges to deeper depth to facilitate quad-HS co-activation and progress to unstable surfaces with ball toss, front step down/single leg squat (week 4-5) progress to higher steps, to train hip to improve valgus do Romanian Dead Lifts (and maybe single leg deadlifts), work on dynamic Q-angle, stand on bosu ball with knee flexed to 30 degrees and ball toss, or same thing single leg kneeling, monster walks, front step downs standing on foam on box, hip strengthening in different positions, single leg squat on box on foam with on/off theraband forces, ball toss on squat on bosu, modified RDL star drill while standing on foam with ball toss, ball toss with single leg stance on bosu (progress with added perturbations), single leg stance on foam while moving medicine ball in different directions – add perturbations to ball, leg press, wall slides, front step downs, front lunges, squats (but be careful), wall slides on a box, leg press and bridging with T-band around thighs, lateral planks with hip abduction, plank with hip extension (progress to stability ball), lateral step down with half foam roller (small preferable). -Advanced Phase (weeks 10-16): Wilk recommends using an alternating day program to not overwork the involved ACL. These components include neuromuscular drills, perturbation training, strengthening, ROM, balances drills, proprioception training and more. Strengthening drills include long arc quads (90-40 degrees of flexion), hamstring exercises in an OKC, Nordic curls, and stability ball bridges. For balance, perform the STAR drill and advance single limb balance activities. Agility drills include single leg balance while performing a dynamic motion with the contralateral limb. Backwards running, light cutting, and varying acceleration speeds are all acceptable. Running is typically started around 10-12 weeks and begins unweighted or in a pool if possible. -Return to Activity Phase (weeks 16-26): Here, the athlete typically begins more sport specific drills. To initiate these higher level exercises, it's important to assure the patient has reached a certain level of knee stability. Knee arthrometer testing and isokinetic testing are commonly used to assess when to advance plyometrics and running. To advance safely, the hamstring/quadriceps ratio should be 66-72% in males and >75% in females. Additionally, the Hop Test can be used and should be 85% of the uninvolved side before progression. As you can see, there is a lot of information regarding the management of ACL injuries, a lot of which is contradictory. You will see many variations of protocols based on the surgeon. Some important basic information to guide your program are the tissue healing time and achievement of the previously listed goals. Whenever you're unsure regarding the development of your treatment program, be sure to consult with another clinician or the surgeon that worked on the patient. What are your thoughts on ACL rehabilitation? Do you have any other interesting evidence? References:
Abbas MM, Abulaban AA, Darwish HH. (2013). Functional outcomes of bone tendon bone versus soft tissue arthroscopic anterior cruciate ligament reconstruction. A comparative study. Saudi Med J;34(2):153-60. Web. 23 Feb 2013. Adams D, et al. (2012). Current concepts for Anterior Cruciate Ligament Reconstruction: A Criterion Based Rehabilitation Progression. JOSPT; 42(7): 601-614. Web. 18 Feb 2013. Baltaci G, Yilmaz G, Atay AO. (2012). The outcomes of anterior cruciate ligament reconstructed and rehabilitated knees versus healthy knees: a functional comparison. Acta Orthop Traumatol Turc;46(3):186-95. Web. 23 Feb 2013. Dave LY, Leong OK, Karim SA, Chong CH. (2013). Tunnel enlargement 5 years after anterior cruciate ligament reconstruction: a radiographic and functional evaluation. Eur J Orthop Surg Traumatol. Web. 24 Feb 2013. Escamilla RF, Macleod TD, Wilk KE, Paulos L, Andrews JR. (2012). Cruciate Ligament Loading During Common Knee Rehabilitation Exercises. Proc Inst Mech Eng H.;226(9):670-80. Web. 23 Feb 2013. Feller J, Webster KE. (2013). Return to sport following anterior cruciate ligament reconstruction. Int Orthop.;37(2): 285-90. Web. 24 Feb 2013. Frank JS, Gambacorta PL. (2013). Anterior cruciate ligament injuries in the skeletally immature athlete: diagnosis and management. J Am Acad Orthop Surg.;21(2):78-87. Web. 24 Feb 2013. Hewett TE, Di Stasi SL, Myer GD. (2013). Current concepts for injury prevention in athletes after anterior cruciate ligament reconstruction. Am J Sports Med. 2013 Jan;41(1):216-24. Web. 24 Feb 2013. Kruse LM, Gray B, Wright RW. (2012). Rehabilitation after anterior cruciate ligament reconstruction: a systematic review. J Bone Joint Surg Am. 3;94(19): 1737-48. Web. 24 Feb 2013. Lepley LK, Palmieri-Smith R. (2012). Effect of Eccentric Strengthening Following Anterior Cruciate Ligament Reconstruction on Quadriceps Strength. J Sport Rehabil. Web. 24 Feb 2013. Kim KM, Croy T, Hertel J, Saliba S. (2010). Effects of neuromuscular electrical stimulation after anterior cruciate ligament reconstruction on quadriceps strength, function, and patient-oriented outcomes: a systematic review. J Orthop Sports Phys Ther.;40(7):383-91. Web. 24 Feb 2013. Lobb R, Tumilty S, Claydon LS. (2012). A review of systematic reviews on anterior cruciate ligament reconstruction rehabilitation. Phys Ther Sport.;13(4):270-8. Web. 23 Feb 2013. Magnussen RA, Pedroza AD, Donaldson CT, Flanigan DC, Kaeding CC. (2013). Time from ACL injury to reconstruction and the prevalence of additional intra-articular pathology: is patient age an important factor? Knee Surg Sports Traumatol Arthrosc. Web. 24 Feb 2013. Manske, Robert C. Postsurgical Orthopedic Sports Rehabilitation: Knee & Shoulder. St. Louis, MO: Mosby Elsevier, 2006. 176-177, 191-192. Print. Markatos K, Kaseta MK, Lallos SN, Korres DS, Efstathopoulos N. (2012). The anatomy of the ACL and its importance in ACL reconstruction. Eur J Orthop Surg Traumatol. Web. 17 Feb 2013. Muller B, Bowman KF Jr, Bedi A. (2013). ACL graft healing and biologics. Clin Sports Med.; 32(1):93-109. Web. 23 Feb 2013. Neumann, Donald. Kinesiology of the Musculoskeletal System: Foundations for Rehabilitation. 2nd edition. St. Louis, MO: Mosby Elsevier, 2010. 535. Print. Nyland J, Mauser N, Caborn DN. (2013). Sports involvement following ACL reconstruction is related to lower extremity neuromuscular adaptations, subjective knee function and health locus of control. Knee Surg Sports Traumatol Arthrosc. Web. 24 Feb 2013. Pan X, Wen H, Wang L, Ge T. (2012). Bone-patellar tendon-bone autograft versus LARS artificial ligament for anterior cruciate ligament reconstruction. Eur J Orthop Surg Traumatol. Web. 24 Feb 2013. Papandreou M, Billis E, Papathanasiou G, Spyropoulos P, Papaioannou N. (2012). Cross-Exercise on Quadriceps Deficit after ACL Reconstruction. J Knee Surg. Web. 24 Feb 2013. Rabuck SJ, Baraga MG, Fu FH. (2013). Anterior cruciate ligament healing and advances in imaging. Clin Sports Med.; 32(1):13-20. Web. 23 Feb 2013. Shelbourne KD, Freeman H, Gray T. (2012). Osteoarthritis after anterior cruciate ligament reconstruction: the importance of regaining and maintaining full range of motion. Sports Health. 2012 Jan;4(1):79-85. Web. 24 Feb 2013. Silverwood R, Gordon JB, Baron R, Walmsley P, Watson J, Wood AM. (2012). Cyclops Lesions in Military Personnel: a reason for delayed return to fitness post anterior cruciate ligament reconstruction. J R Nav Med Serv; ;98(3):3-5. Web. 23 Feb 2013. Sutton KM, Bullock JM. (2013). Anterior cruciate ligament rupture: differences between males and females. J Am Acad Orthop Surg.;21(1):41-50. Web. 24 Feb 2013. Van Ginckel A, Verdonk P, Victor J, Witvrouw E. (2013). Cartilage Status in Relation to Return to Sports After Anterior Cruciate Ligament Reconstruction. Am J Sports Med. Web. 24 Feb 2013. Wilk, K. (Performer) (2012). Current concepts in the treatment of acl injuries parts 1-3. Rehab Webinars. [Audio podcast]. Retrieved from www.rehabwebinars.com Xu M, Gao S, Zeng C, Han R, Sun J, Li H, Xiong Y, Lei G. (2013). Outcomes of Anterior Cruciate Ligament Reconstruction Using Single-Bundle Versus Double-Bundle Technique: Meta-analysis of 19 Randomized Controlled Trials. Arthroscopy.;29(2):357-65. Web. 24 Feb 2013. Zelić Z, Jovanović S, Wertheimer V, Sarić G, Biuk E, Gulan G. (2012). Results of surgical reconstruction of the anterior cruciate ligament. Coll Antropol;36(1):201-6. Web. 23 Feb 2013. Zhu W, Wang D, Han Y, Zhang N, Zeng Y. (2012). Anterior cruciate ligament (ACL) autograft reconstruction with hamstring tendons: clinical research among three rehabilitation procedures. Eur J Orthop Surg Traumatol. Web. 24 Feb 2013.
11 Comments
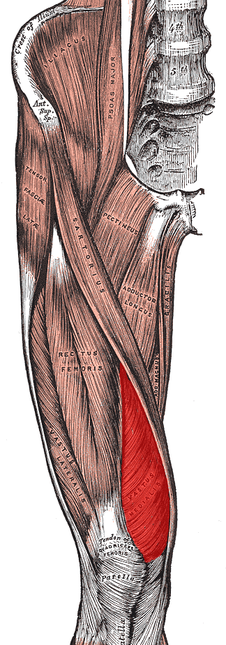
No matter where you do your rotations or practice physical therapy, you are bound to work with both people who target the VMO with their interventions and people who think it's impossible to do so. Following trauma, knee surgery, or patellofemoral pain syndrome, many practitioners claim selective atrophy and weakness of the VMO relative to the rest of the quadriceps. This becomes a focus of several interventions in the patient's care plan. Due to the controversial state of this case, we thought we would do a review on the isolation of the vastus medialis muscle. Let's start out with reviewing a little anatomy. The quadriceps is made up of four parts: rectus femoris, vastus lateralis, vastus medialis, and vastus intermedius. Rectus Femoris Origin: -Anteroinferior iliac spine (straight head), groove above rim of acetabulum (reflected head) Vastus Lateralis Origin: -Proximal part of intertrochanteric line, anterior and inferior borders of greater trochanter, lateral lip of the gluteal tuberosity, proximal half of lateral lip of linea aspera, and lateral intermuscular septum Vastus Intermedius Origin: -Anterior and lateral surfaces of the proximal 2/3 of the body of the femur, distal half of the linea aspera, and lateral intermuscular septum Vastus Medialis Origin: -Distal half of the intertrochanteric line, medial lip of the linea aspera, proximal part of the medial supracondylar line, tendons of the adductor longus and adductor magnus and medial intermuscular septum Common Insertion: -Proximal border of the patella and through the patellar ligament to the tuberosity of the tibia Common Innervation: -Femoral nerve (L2-4) Common Action: -Extends the knee (rectus femoris also flexes the hip) *(Kendall et al, 2005) One way of analyzing this controversial issue is anatomically. Hubbard et al reviewed the anatomy of cadavers to enhance the interpretation of VMO isolation. The vastus medialis is commonly broken down into two components: vastus medialis longus (VML) and vastus medialis oblique (VMO). It is thought that the VMO is responsible for medial patellar tracking, due to its oblique fiber orientation. In the study, the VMO was unable to be found separated from the VML, meaning the VMO was not found to be a muscle independently. Any attempted separation required destruction of muscle fibers. Upon review of innervation, many studies in the past have come across independent innervation, leading to the hypothesis that the VMO was a separate muscle. However, these nerves have been found to be superficial separations from the femoral nerve leading to distal motor units, sensory contribution from the saphenous nerve, or penetrating innervation for the knee capsule. The authors argue that without isolated innervation, the VMO cannot be activated independently (the patella cannot solely be pulled medially); it is the activity of the femoral nerve the stimulates the entire vastus medialis to contract and extend the knee while pulling the patella medially. Historically, many people were under the impression that the rectus femoris was the prime knee extensor. They thought the vastus medialis was responsible for the final 15 degrees of knee extension due to the inadequacies of the rectus femoris (Lieb & Perry, 1968). Boucher et al looked at the vastus medialis activity in patients with patellofemoral pain syndrome. They found that the vastus medialis and vastus lateralis were not more active in terminal extension. However, they did find that in patients with PFPS, there was a decreased VM:VL ratio compared to the control group. These differences were found to be attributable to a mechanical disadvantage (greater Q-angle); when the Q-angle was decreased, the ratio returned to normal. This same difference in VM and VL ratio was found in another study to be related to mechanical factors as well (Souza & Gross, 2001). Additionally, the authors discovered that isotonic quadriceps contractions elicited larger VM:VL EMG activity compared to isometric contractions. This may influence the treatment plan for patients with pathologies, such as patellofemoral pain syndrome. Ng et al. and Cowan et al. also came across this difference in VM and VL ratio with EMG activity. In fact, the authors were able to normalize the EMG activity with use of therapeutic exercise + biofeedback. Biofeedback has been found to be a useful component in motor retraining, especially regarding earlier activation of the VMO (Cowan et al., 2002). A delay of as little as 5 ms of the VMO relative to the VL can increase the compression forces on the lateral patellafemoral joint (Boling et al, 2006). Powers described a difference between VM and VL activity as well; however, it was attributed to patellar malalignment. Decreased VM activity was not found to be the cause of abnormal patellar tracking or patellar tilt.
As it has probably become obvious, VMO isolation is a controversial issue with many conflicting studies. Thein Brody & Hall do an excellent job summarizing the research, while offering their own opinion. Regarding measuring EMG amplitude, they point out that EMG measures electrical activity, not force production. To truly assess the effects the VMO has on the patella, one must consider fiber orientation, and cross-sectional area. The authors then return back to an anatomical discussion, stating an isolated VMO contraction has never been displayed. This suggests training the VMO independently to improve timing may not be possible. In fact, VMO:VL timing has been shown to improve after training the quadriceps muscle as a whole. So what are your thoughts and what have your experiences been on this issue? Should we be spending so much time trying to isolate the VMO for rehabilitation or focus on a more general method? Let us know! References:
Boling MC, Bolgla LA, Mattacola CG, Uhl TL, Hosey RG. "Outcomes of a weight-bearing rehabilitation program for patients diagnosed with patellofemoral pain syndrome." Arch Phys Med Rehabil. 2006 Nov;87(11):1428-35. Boucher JP, King MA, Lefebvre R, Pépin A. "Quadriceps femoris muscle activity in patellofemoral pain syndrome." Am J Sports Med. 1992 Sep-Oct;20(5):527-32. Web. 17 Nov 2012. Brody LT & Hall CM. (2011). Therapeutic Exercise: Moving Toward Function. (3rd ed., pp. 530-531). Baltimore: Lippincott Williams & Wilkins. Cowan SM, Bennell KL, Hodges PW, Crossley KM, McConnell J. "Delayed onset of electromyographic activity of vastus medialis obliquus relative to vastus lateralis in subjects with patellofemoral pain syndrome." Arch Phys Med Rehabil. 2001 Feb;82(2):183-9. Web. 26 Nov 2012. Cowan SM, Bennell KL, Crossley KM, Hodges PW, McConnell J. "Physical therapy alters recruitment of the vasti in patellofemoral pain syndrome." Med Sci Sports Exerc. 2002 Dec;34(12):1879-85. Web. 26 Nov 2012. Gilleard W, McConnell J, Parsons D. "The effect of patellar taping on the onset of vastus medialis obliquus and vastus lateralis muscle activity in persons with patellofemoral pain." Phys Ther. 1998 Jan;78(1):25-32. Web. 25 Nov 2012. Hubbard JK, Sampson HW, Elledge JR. "Prevalence and morphology of the vastus medialis oblique muscle in human cadavers." Anat Rec. 1997 Sep;249(1):135-42. Web. 25 Nov 2012. Kendall F, McCreary E, Provance P, Rodgers M, & Romani W. (2005). Muscles: Testing and Function with Posture and Pain. (5th ed., p. 420). Baltimore: Lippincott Williams & Wilkins. Lieb FJ, Perry J. "Quadriceps function. An anatomical and mechanical study using amputated limbs." J Bone Joint Surg Am. 1968 Dec;50(8):1535-48. Web. 17 Nov 2012. Ng GY, Zhang AQ, Li CK. "Biofeedback exercise improved the EMG activity ratio of the medial and lateral vasti muscles in subjects with patellofemoral pain syndrome." J Electromyogr Kinesiol. 2008 Feb;18(1):128-33. Web. 17 Nov 2012. Powers CM, Landel R, Perry J. "Timing and intensity of vastus muscle activity during functional activities in subjects with and without patellofemoral pain." Phys Ther. 1996 Sep;76(9):946-55. Web. 25 Nov 2012. Powers CM. "Patellar kinematics, part I: the influence of vastus muscle activity in subjects with and without patellofemoral pain." Phys Ther. 2000 Oct;80(10):956-64. Web. 25 Nov 2012. Smith TO, Bowyer D, Dixon J, Stephenson R, Chester R, Donell ST. "Can vastus medialis oblique be preferentially activated? A systematic review of electromyographic studies." Physiother Theory Pract. 2009 Feb;25(2):69-98. Web. 25 Nov 2012. Souza DR, Gross MT. "Comparison of vastus medialis obliquus: vastus lateralis muscle integrated electromyographic ratios between healthy subjects and patients with patellofemoral pain." Phys Ther. 1991 Apr;71(4):310-6. Web. 25 Nov 2012. Stensdotter AK, Hodges P, Ohberg F, Häger-Ross C. "Quadriceps EMG in open and closed kinetic chain tasks in women with patellofemoral pain." J Mot Behav. 2007 May;39(3):194-202. Web. 26 Nov 2012. |
Copyright © The Student Physical Therapist LLC 2023


 RSS Feed
RSS Feed